On January 25th, 2023, Professor Guangmin Yao’s group published an article entitled“Piericones A and B as Potent Antithrombotics: Nanomolar Noncompetitive Protein Disulfide Isomerase Inhibitors with an Unexpected Chemical Architecture” on theJournal of the American Chemical Society(2023,145(5), 3196–3203.IF: 16.383),the topjournal in the field of Medicinal Chemistry. The article reportednanomolar noncompetitive protein disulfide isomerase inhibitors with an unexpected chemical architecture.
Thrombotic-related diseases such as ischemic stroke and myocardial infarction are common cardiovascular and cerebrovascular disorders that seriously endanger human health with a high morbidity and fatality rate. Platelet activation and aggregation are critical steps during thrombus formation, however, antiplatelet agents such as aspirin and clopidogrel are commonly associated with increased bleeding risk and/or treatment failure to various degrees.
Protein disulfide isomerase (PDI), the prototypic member of the thiol isomerase family, catalyzes the oxidation, reduction, and isomerization of protein disulfide bonds.Extracellular PDI plays a critical role in thrombosis by regulating platelet aggregation and fibrin generation. Inhibition of extracellular PDI is a promising strategy to develop novel antithrombotics without bleeding risks.
In a course of searching for novel antithrombotics from traditional Chinese medicines,six potent PDI inhibitorswere isolatedfrom the leaves ofPieris japonica(Ericaceae),including piericones A−D (1−4)with two unprecedentedchemicalarchitectures.Piericones A (1) and B (2) were determined as unique hexahydro-10H-furo[2′′,3′′: 3′,4′]furo[2′,3′:3,4]furo[2,3-c]pyran-6(6aH)-one bridged bisdihydrochalcones, featuring an unprecedented 3,6,10,15-tetraoxa-tetracyclo [7.6.0.04,9.01,12]pentadecane motif with nine contiguous stereogenic centers. Piericones C (3) and D (4) are unprecedented dihydrochalcone-substituted trihydroxytetrahydrofuro[3,4-b]furan-4(2H)-ones featuring a unique 2,7-dioxabicyclo[3.3.0]octane core.Their structures were elucidated by spectroscopic data analysis, chemical methods, quantum13C NMR DP4+ and ECD calculations, and single-crystal X-ray diffraction analysis.Their biosynthetic pathways were proposedto include a key intermolecular aldol reaction and an intramolecular 1,2-migration reaction.
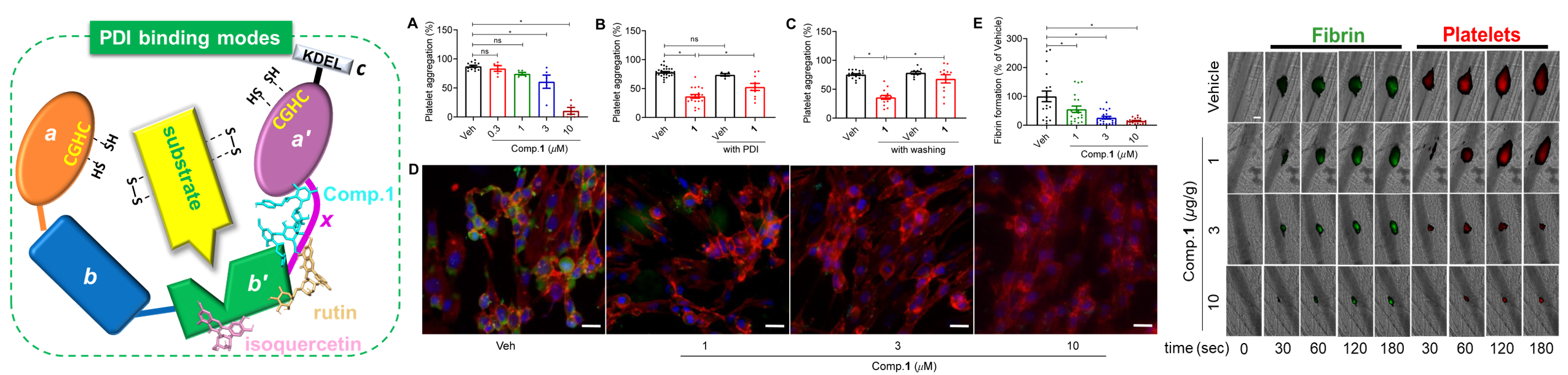
Inhibitors1,2, and4showed nanomolar inhibitory activities with IC50values of 0.15 ± 0.04, 0.23 ± 0.03, and0.50 ± 0.15μM, respectively, which are about 41.8, 20.9, and 12.5 times lower than that of isoquercetin (the positive control), respectively.Importantly,the most potent inhibitor1showed specificity for PDI, rather than other PDI family members such as endoplasmic reticulum protein 5 (ERp5), ERp57, and ERp72.In this study, the inhibitor types andKiofPDI inhibitors were determined for the first time by the enzyme kinetic assay. The doublereciprocal Lineweaver-Burk plots intersecting at thex-axis revealed that1,2, and isoquercetin were noncompetitive PDI inhibitors, with theKivalues of 0.73 ± 0.03, 0.72 ± 0.05, and 19.90 ± 3.29μΜ, respectively.The molecular docking and molecular dynamics simulation revealed that compound1show binding affinity to residuesat the interface ofb′xa′ domains, compounds2−5bind to bothb′ domain andxlinker, whereas,6and isoquercetin bind to theb′ domain.Piericone A (1) significantly inhibitedin vitroplatelet aggregation and fibrin formation, andin vivothrombus formationviathe inhibition of extracellular PDI.Compound1did not increase the risk of bleedingin vivoanddid not present cytotoxic effects onplatelets, endothelial cells, and isolated neutrophils.
These findings should provide useful clues for discovery of novel high-efficacy PDI inhibitors based on the unprecedented chemical architectures, and trigger further investigations on the total synthesis, biosynthesis, and pharmacology of1and2as promising leads for new antithrombotics.Post-doctor Guijuan Zheng in Professor Guangmin Yao’s grop is the first author, and Professors Guangmin Yao and Chao Fang are the co-corresponding authors.
Interestingly, althoughasebogenin (7)has no significant PDI inhibition,it inhibited GPVI-induced platelet aggregation, granule secretion, integrin activation, and intracellular Ca2+mobilization. Asebogenin inhibited NETs formation induced by proinflammatory stimulus in neutrophils. Mechanistically, compound7attenuated Syk phosphorylation and related downstream signaling events by directly targeting to Syk. Further, asebogenin inhibited both arterial thrombosis as determined by reduced platelet accumulation and fibrin generation in the laser injury-induced thrombosis model and venous thrombosis model induced by IVC stenosis.The related results were published on theBritish Journal of Pharmacology(2023,180, 287-307.IF 9.473), the top journal in the field of pharmacology, entitled“Asebogenin suppresses thrombus formation via inhibition of Syk phosphorylation”.Professors Chao Fang and Guangmin Yao are the co-corresponding authors.
Three Chinese patents (application numbers: 202211294113.8, 202210087140.1, and 20221141485.7) have been applied based on the above results.
This work was supported by the National Natural Science Foundation of China, National Key R&D Program of China, Natural Science Fund for Distinguished Young Scholars of Hubei Province, and China Postdoctoral Science Foundation, respectively.
Article links:
https://doi.org/10.1021/jacs.2c12963
https://bpspubs.onlinelibrary.wiley.com/doi/10.1111/bph.15964



