The protein encoding the RAS gene is a key molecule involved in cell signal transduction, regulating cell proliferation and differentiation. When mutation occurs, the RAS protein remains in an activated state, leading to disrupted signal transduction and sustained cell proliferation leading to the occurrence of tumors. According to statistics, 30% of human malignant tumors are related to RAS gene mutations, including lung cancer, rectal cancer and pancreatic cancer. However, due to the relatively "smooth" surface of RAS protein and the lack of "pockets" suitable for small molecule binding, the development of drugs targeting RAS protein has progressed slowly. The development of drugs targeting RAS proteins with small molecules still faces challenges.
Prenyl modification is a kind of hydrophobic chemical modification group with C5 as the main skeleton, which is different from methylation modification. It modifies RNA in oligomerization and protein in poly polymerization. Recently, studies have reported that such modifications are associated with COVID-19 (Science 2021, 374 (6567), eabj3624.) and neurodegenerative diseases (J Immunol, 2002, 168: 4087-94 J Biol Chem, 2013, 288: 35952-60) is closely related. However, there have been no reports of using chemical biology methods to intervene and regulate it.

Recently, Professor Wang's research group published the new progress in this field (Degradation of RAS Proteins Targeting the Posttranslational Prenl Modifications via Cascade Azidation/Fluorination and Click Reaction, J. Med. Chem. 2023, ASAP. The co-first authors are Dr. Zhou Hongling, graduates Gan Youfang and Li Yuanyuan, the only corresponding author is Professor Wang, and other authors include graduates Chen Xiaoqian and Guo Yuyang. The research has received support from the Startup Fund of Huazhong University of Science and Technology.
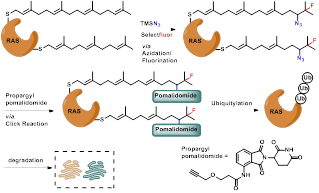
Figure 1 Degradation strategy targeting prenyl modification in RAS.
Inspired by the classic protein targeted degradation chimeric technique (PROTAC), this study developed a degradation strategy for targeting post translational prenyl modified RAS based on previous research. After being validated at the small molecule level, the azidation/fluoridation was further confirmed in fluorescence labeling of proteins such as Lysozyme, HRAS, and KRAS, with excellent selectivity and efficiency (Figure 2). Both the strategy of azidation/fluoridation and the direct fluorescent probe method based on PTAD have achieved the expected results (Figure 2).
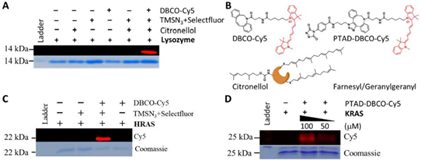
Figure 2 Fluorescence labeling of proteins targeting prenyl PTM
By means of proteomics and functional probes developed by the research group, chemical biological methods were used to analyze prenyl modification of RAS protein. Through a comparative study of three different research strategies targeting prenyl modification, it was found that Cys51, Cys80, Cys118, and Cys186 were chemically modified with prenyl, although the content identified by different methods varied (Figure 3). This result is consistent with the literature reporting that prenyl modification is often located in Motif CaaX, as shown in Figure 3. Especially, the crystal structure analysis of the three sites Cys80, Cys118, and Cys186 all located in the surface region of the protein, laying the foundation for its targeted biodegradability.
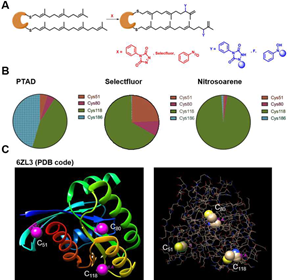
Figure 3 Identification and confirmation of prenyl modification in RAS protein.
Finally, this study proposes a novel targeted degradation strategy for post translational prenyl modification of proteins. Unlike traditional and classic methods for screening and optimizing ligands that bind to proteins, the new targeted protein chimerism method targeting post-translational modification, only requiring development of biocompatible reactions and specifically recognized antibodies. This approach provides new insights into the regulation of numerous post translational modified proteins, such as hyperphosphorylated tau (pTau) protein entanglement, which is one of the two classic pathological features of Alzheimer's disease (AD). Research on phosphorylation and other PTMs provides new insights.
Specifically, we explored degradation experiments on different cell lines using well-designed and chemically synthesized probes. (Figure 4). Based on this approach, this strategy exhibits good degradation efficiency in HeLa and HT-29 cell lines. Other cell lines such as A549 and MCF-7 also have certain application potential. In summary, the PROTAC technology, which targeting RAS’s prenyl PTM, has expanded the PROTAC toolsets and exhibits application potential.
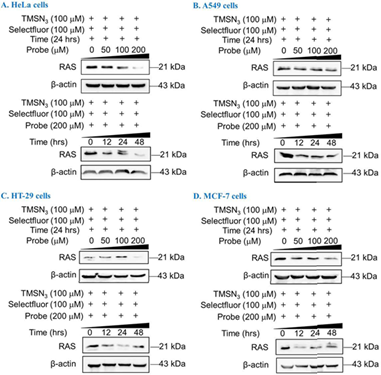
Figure 4 Targeted degradation of RAS in different cell lines
Original website:
DOI: 10.1021/acs.jmedchem.2c01721.



